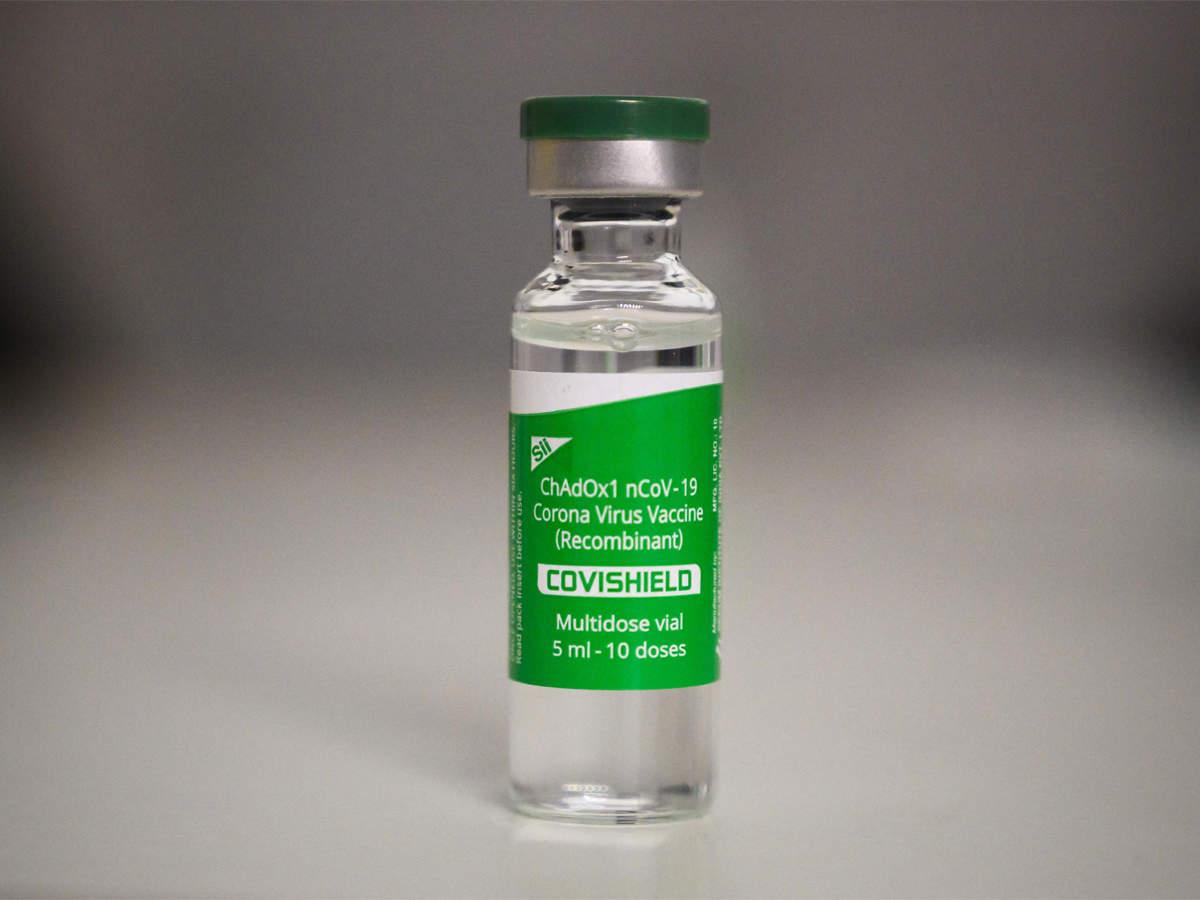
The Covishield version of AstraZeneca, produced by Pune-based Serum Institute of India (SII), has not been included in the list.
Domestically-manufactured Covishield, a version of the AstraZeneca vaccine, has been excluded from the list of anti-COVID-19 jabs that are eligible to avail the European Union (EU)’s ‘Green Pass’.
~~Featured COVID-19 articles~~
- Don’t Panic, here is how to BOOST your immune system to BEAT colds, flu and coronavirus 🙂
- Is ordering food for takeout or home delivery safe during corona virus outbreak?
- 🙏Pls Don’t Scare People and Kids About Corona! Majority Recover. Educate yourself and them about FACTS from W.H.O
- Options for Online Learning handy skills, new languages, short courses and knowedge gain while stuck at home
- COVID-19: Who is coping better? Indian Wives or NRI Wives? Lessons learnt in lockdown without Maid #ShareTheLoad
~~Featured articles end here~~
Green Pass, which would be available from July 1 onwards, can be used for unrestricted movement in all EU member states for business and tourism purposes. The pass would be granted to those who have been inoculated with any of the vaccines that have been approved by the European Medicines Agency (EMA).
——Advertisement starts here ——
 NRIForShaadi.com World’s #1 App for NRI Matrimony. Thousands of members near your GPS Location. Download from NRIApps.com
NRIForShaadi.com World’s #1 App for NRI Matrimony. Thousands of members near your GPS Location. Download from NRIApps.com ———Advertisement ends here ———
The EMA has so far cleared four vaccines – Comirnaty (Pfizer/BioNTech), Moderna, Vaxzervria (AstraZeneca), Janssen (Johnson & Johnson).
According to schengenvisainfo.com – EU’s official portal for visa-related information – only the Vaxzervia version of AstraZeneca vaccine – manufactured in the UK and other sites in Europe – is eligible for Green Pass. The Covishield version of AstraZeneca, produced by Pune-based Serum Institute of India (SII), has not been included in the list.
Continue reading at https://www.moneycontrol.com/news/trends/travel-trends/eu-excludes-anti-covid-19-vaccine-covishield-from-green-pass-list-7094371.html